Importance Of Buffer Solution In Pharmacy
Buffer capacity is def. Definition of a Buffer which you can easily find in every book or site is.
 Applications Of Buffer In Industries
Applications Of Buffer In Industries
Acidic buffer solutions are thosethat have strong acids and weak bases as their components.
Importance of buffer solution in pharmacy. For example a borate buffer because of its toxic. Acidic buffers are used to neutralize alkaline solutions because of the weak acids in the alkaline solution. Buffer solutions are necessary to keep the correct pH for enzymes in many organisms to work.
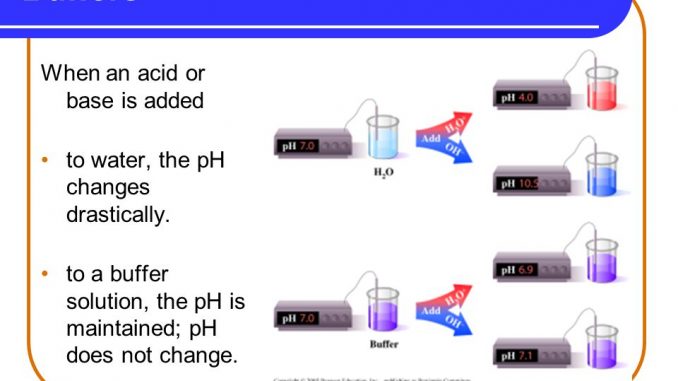
Buffer solutions are able to resist a significant change in pH when a limited concentration of acid or. In order for a particular reaction to occur or to occur at an appropriate rate the pH of the reaction medium must be controlled. Buffer solutions are solutions that resist changes in pH upon addition of small amounts of acid or base or upon dilution.
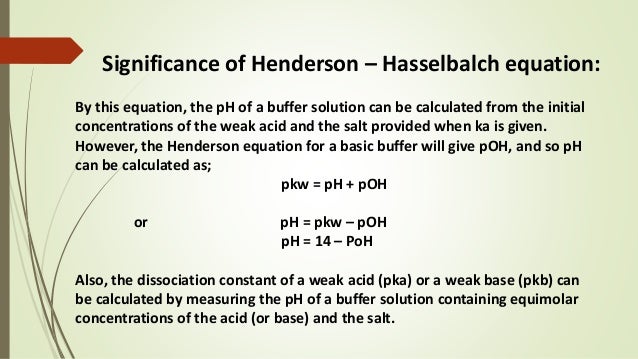
A solution which resists the change in its pH value even on the addition of a small amount of strong acid or base is called a buffer solution or buffer. A buffer solution contains an acid and its conjugate base or a base and its conjugate acid. A buffer capacity of 001 to 01 is generally adequate.
Buffer solutions are important functions throughout the body. Examine the types and uses of pharmaceutical solutions as oral drug delivery systems. A buffered solution is one that resists changes in its pH when small amounts of acid or base are added or when the solution is diluted.
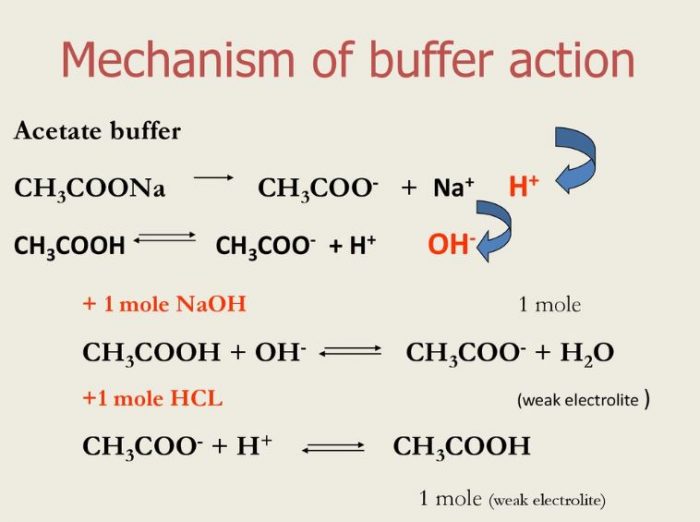
Buffer solutions are important because they help to neutralize a reaction to a certain extent. Mixture of acetic acid CH 3 COOH and Sodium acetate CH 3 COONa in water. Stability of the drug and buffer on aging.
The addition of any compound to a solution will affect the isotonicity since isotonicity is a property of the number of particles in solution. Importance of Buffer SolutionBy Mike Charmaine eHow Contributorupdated. Buffer solutions are solutions in water that mark the combination of acids and bases.
Buffers are used in pharmaceutical products for 2 puposes. Biological Importance of Buffers. 1 chapter 1 Pharmaceutical solutions for oral administration In this chapter we will.
Such control is provided by buffer solutions which are solutions that maintain a particular pH. They help in a neutralization reaction to a certain extent. 226 Importance of Buffers Many chemical reactions are affected by the acidity of the solution in which they occur.
A buffer solution resists change in pH upon addition of any compound that tend to alter hydrogen ion concentration. BUFFERS IN PHARMACEUTICAL SYSTEMS Solid Dosage FormsBuffers have been widely in solid dosage forms such as tablets capsules and powders for controlling the PH of the environment around the solid particles. So the osmotic pressure of a solution will be affected not only by the drug but also by any buffer compounds that are included in the formulation.
This prevents the solutions becoming too acidic and spoiling the product. Many enzymes work only under very precise conditions. These can be any weak acidweak base pair but are usually a conjugate acidconjugate base pair.
If the pH strays too far out of the margin the enzymes slow or stop working and can denature thus permanently disabling its catalytic activity. Availability and cost of chemicals Sterility of the final solution. This has practical application for the drugs that have dissolution rate limited absorption from unbuffered solutions.
The buffer is used to maintain a specific pH of the solution it is used in the analysis and manufacture of various manufacturing processes such as food processing electroplating manufacture of medicines especially injection ear drops eye droplets suspension dissolution of tablets etc. Many dyeing processes use buffers to maintain the correct pH for various dyes. The specific roles of each of these formulation excipients will be described later in this chapter.
In the textile Industry. June 17 2010Buffer solutions are solutions in water that mark the combination of acids and basesThey help in neutralization reaction to a certain extent. D-Other factors of some importance in the choice of a pharmaceutical buffer include availability of chemicals sterility of the final solution stability of the drug and buffer on aging cost of materials and freedom from toxicity.
To adjust the pH of product for maximum stability. Buffer solutions are added before fermentation begins. Buffer solutions contain an acid to react with added OH and a base to react with added H.
17 of Pharmaceutical buffer solutionsPreparation Factors of some importance in the choice of pharmaceutical buffer include. What Is the Importance of Buffers in Pharmacy. Acidic buffer solutions are those that have strong acids and weak bases as their components.
Http Courseware Cutm Ac In Wp Content Uploads 2020 06 Role Of Buffers In Pharmacy Pdf
 How Does Titration Determine Concentration Chemistry Laboratory Techniques Concentration
How Does Titration Determine Concentration Chemistry Laboratory Techniques Concentration
 Log In Tumblr Pediatric Nursing Nursing School Tips Nurse
Log In Tumblr Pediatric Nursing Nursing School Tips Nurse
 Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
 Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
 Nursing Schools Studying Ekg Collegescholarshipsforadultswebsite Nursing School Notes Nursing School Survival Nursing School
Nursing Schools Studying Ekg Collegescholarshipsforadultswebsite Nursing School Notes Nursing School Survival Nursing School
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 Free Iv Fluid Guide And Cheat Sheet 2020 Update Nursing School Prerequisites Nursing School Scholarships Best Nursing Schools
Free Iv Fluid Guide And Cheat Sheet 2020 Update Nursing School Prerequisites Nursing School Scholarships Best Nursing Schools
 Pharmacy Design Pharmacy Design Office Floor Plan Medical Office Design
Pharmacy Design Pharmacy Design Office Floor Plan Medical Office Design
 How Is It Important To Identify The Environmental Isolates By Www Pharmaguideline Com Environment Pharmaceutical Home Appliances
How Is It Important To Identify The Environmental Isolates By Www Pharmaguideline Com Environment Pharmaceutical Home Appliances
What Is The Role Of A Buffer In A Pharmacy Quora
 The Presence Of Order Picking Systems And Case Picking Systems Is Beneficial For Logistics Inventory Management Software System Business Tips
The Presence Of Order Picking Systems And Case Picking Systems Is Beneficial For Logistics Inventory Management Software System Business Tips
 Temperature Mapping In Pharmaceuticals Pharmacy Student Buffer Solution Volume Of A Cylinder
Temperature Mapping In Pharmaceuticals Pharmacy Student Buffer Solution Volume Of A Cylinder
 Importance Of Buffer Solution In Pharmacy Pharmacywalls
Importance Of Buffer Solution In Pharmacy Pharmacywalls