Definition Of Buffer In Biology
Chemistry A substance that prevents change in the acidity of a solution when an acid or base is added to the solution or when the solution is diluted. Buffers are used during cell lysis breakdown protein separation protein transfer and blocking phases.
 Pin De Biology 4u En Simple Biology Videos
Pin De Biology 4u En Simple Biology Videos
An example of a common buffer is a solution of acetic acid CH 3 COOH and sodium acetate.

Definition of buffer in biology. When an acid or alkali has added the pH of the solution changes in the absence of buffers. Carbon dioxide is part of a prominent buffer system in the human body. In nature there are many systems that use buffering for pH regulation.
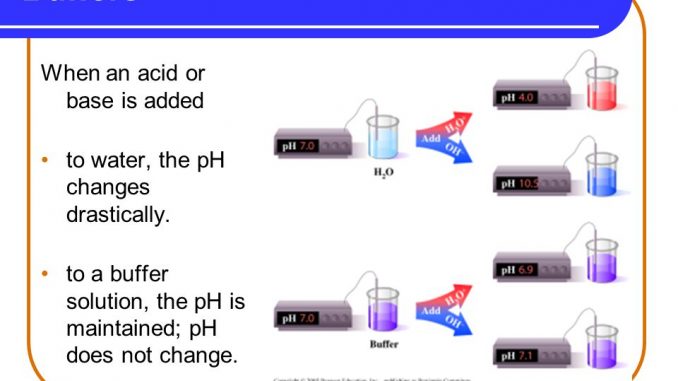
Buffers are used to make solutions of known pH especially for instrument calibration purposes. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A capacity of 1 when 1 mol of acid or alkali is added to 1 litre causes a pH fall or rise of 1 pH unit.
Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. Buffers that are continuous around the perimeter or along the length of a sensitive habitat. Buffer in chemistry solution usually containing an acid and a base or a salt that tends to maintain a constant hydrogen ion concentration.
2 biochemistry An ionic compound that when added to a solution neutralizes both acids and bases without significantly changing the original acidity or alkalinity of a solution. Biological buffers are organic substances that maintain a constant pH over a given range by neutralizing the effects of hydrogen ions. Return to Search Page.
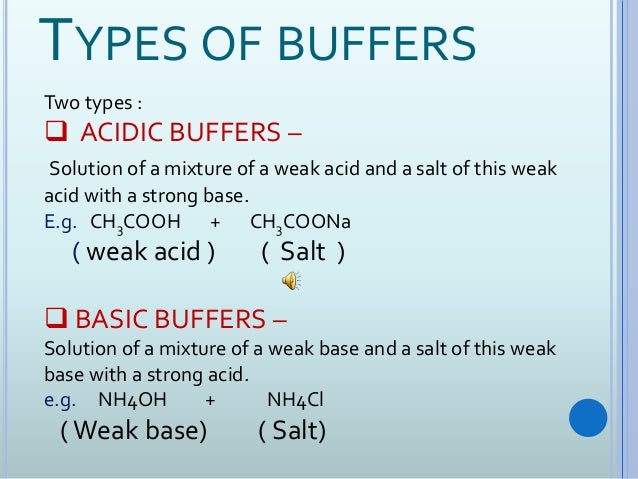
Buffers are the mixture of weak acids and their salts of strong bases or the mixture of weak bases and their salts of strong acidsBuffers help to maintain a normal pH of the biological systems. In water solution sodium acetate is completely dissociated into sodium Na and acetate CH 3 COO - ions. Buffer capacity A measure of the ability of a solution to maintain its pH in the face of the addition of acid or alkali.
It keeps the pH within the proper range. A substance or mixture of substances as bicarbonates and some proteins in biological fluids that in solution tends to stabilize the hydrogen-ion concentration by neutralizing within limits both acids and bases. This buffer system involves carbonic acid H 2 CO 3 and bicarbonate HCO 3 anion.
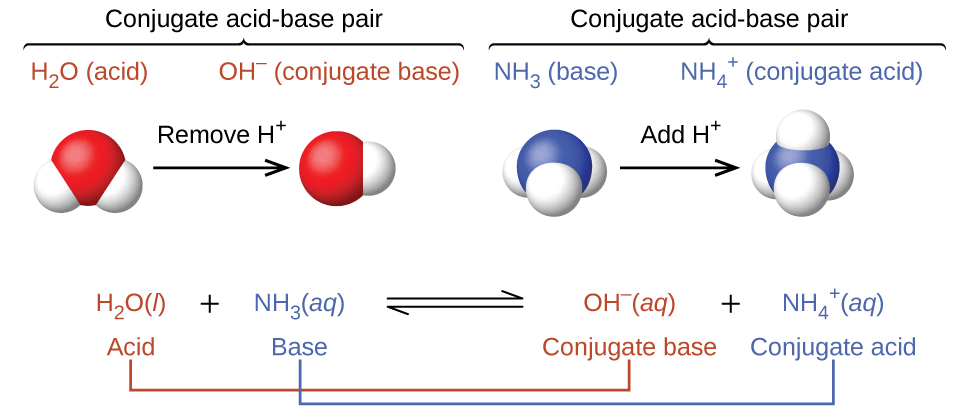
In this way a biological buffer helps maintain the body at the correct pH so that biochemical processes continue to run optimally. If too much H enters the body bicarbonate will combine with the H to create carbonic acid and limit the decrease in pH. Buffer A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate baseor a weak baseand its conjugate acid.
A solution containing either a weak acid and a conjugate base or a weak base and a conjugate acid used to stabilize the pH of a liquid upon dilution. Sample Preparation To carry out Western blot analysis you first need a tissue sample. A buffer is an aqueous solution used to keep the pH of a solution nearly constant.
An area designed to separate. Buffer a weak acid or base that can react with strong acids or bases to help prevent sharp sudden changes in pH THIS SET IS OFTEN IN FOLDERS WITH. Most buffers consist of a weak acid and a weak base.
Cological buffers are protected zones established around sensitive or critical areas such as wildlife breeding or hibernation habitats streams and wetlands to lessen the impacts of human activity and. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. It has the property that the pH of the solution changes very little when a small.
Its pH changes very little when a small amount of strong acid or base is added to it. Ions are atoms or molecules that have lost or gained one or more electrons. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions.
Buffers are commonly used in research labs especially in applications involving protein electrophoresis polymerase chain reaction and Western blotting. Buffers are the mixtures of weak acids and their salts of strong bases or strong acids and their salts of weak bases. Buffer zone definition is - a neutral area separating conflicting forces.
Buffer a chemical substance which has the capacity to bond to H ions removing them from solution when their concentration begins to rise and releasing H ions when their concentration begins to fall. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Biology I - Chapter 2-1.
How to use buffer zone in a sentence. For example the bicarbonate buffering system is used to regulate the pH of blood. Medical Definition of buffer Entry 1 of 2 1.
In this way buffers stabilize the pH of biological solutions and are thus important in maintaining HOMEOSTASIS. 1 chemistry A buffer solution.
 Common Ion Effect Buffer Solution And Solubility Product Buffer Solution Solubility Solutions
Common Ion Effect Buffer Solution And Solubility Product Buffer Solution Solubility Solutions
 Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
 Why Isotopes Are Unstable Chemistry Basics Chemistry Science Student
Why Isotopes Are Unstable Chemistry Basics Chemistry Science Student
 Lec 9 Level 4 De Biological Buffer
Lec 9 Level 4 De Biological Buffer
 Genetic Testing Hopes Biology Labs Biology Classroom Biochemistry
Genetic Testing Hopes Biology Labs Biology Classroom Biochemistry
 This Video Is First Part Of Uv Spectroscopy It Covers Electromagnetic Spectrum Beer La Teaching Chemistry Electromagnetic Spectrum Medical Laboratory Science
This Video Is First Part Of Uv Spectroscopy It Covers Electromagnetic Spectrum Beer La Teaching Chemistry Electromagnetic Spectrum Medical Laboratory Science
 Buffers Definition Overview Expii
Buffers Definition Overview Expii
 Common Ion Effect Chemistry Digital Common
Common Ion Effect Chemistry Digital Common
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 Pin By Arman Afshar On Data Molecular Biology Biology Teaching Biology
Pin By Arman Afshar On Data Molecular Biology Biology Teaching Biology
 Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
 Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry